# Understanding Creatine Transporter Deficiency
Important Note
This article addresses a health topic. It is not intended for self-diagnosis nor does it replace a doctor's diagnosis. Please refer to our General and Medical Disclaimer.
# Creatine
# General Information
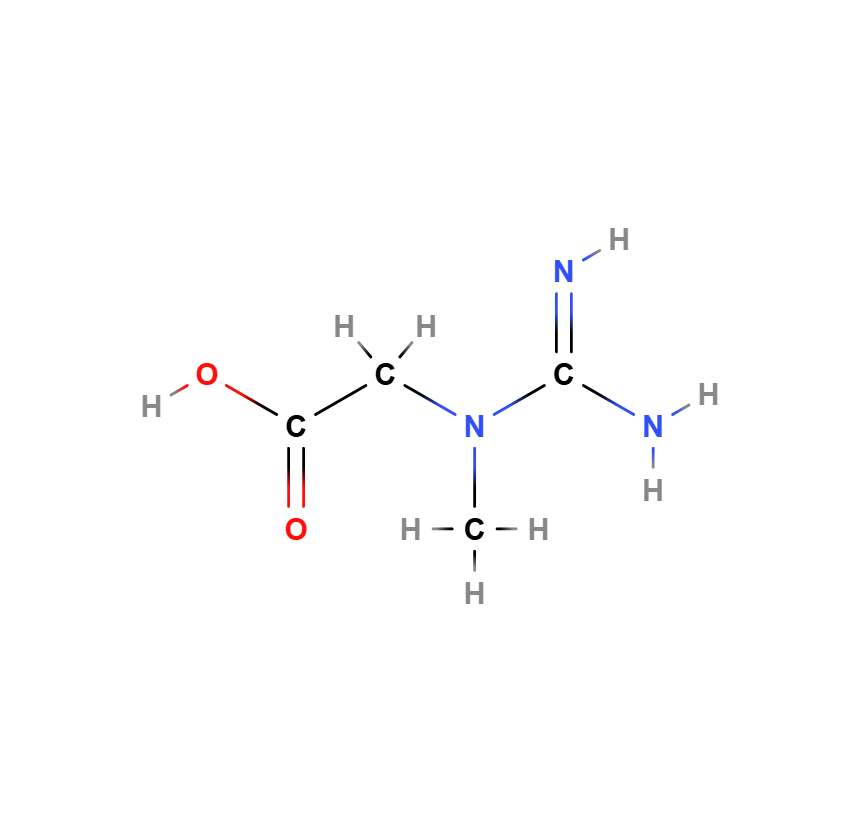
Creatine (C₄H₉N₃O₂) is a nitrogen-containing organic acid formed from the three proteinogenic amino acids arginine, glycine, and methionine. Its main function for the body is to supply cells with high-energy phosphates. It plays an essential role in the energy metabolism of cells, particularly in tissues with high energy demands such as muscle and nerve cells. Its chemical structure contains a guanidine group and a carboxyl group, which are critical for its biological properties.
The structural formula shows:
- A guanidine group (-NH-C(=NH)-NH₂), which is crucial for the energetic properties of creatine.
- A carboxyl group (-COO⁻), which facilitates water solubility and absorption in the body.
# Physiological Dependence on Transporter Proteins
It is critical that creatine, due to its chemical structure, cannot pass through the bilayer lipid membrane of the cell. Creatine is a hydrophilic molecule with polar functional groups, particularly the guanidinium and carboxyl groups. These groups interact strongly with water, preventing direct diffusion through the lipophilic membrane. The cell membrane consists of a phospholipid bilayer, with the hydrophobic fatty acid chains acting as a barrier for polar and charged molecules. Due to these physicochemical properties, creatine cannot pass through the membrane freely. To fulfill its multifaceted physiological tasks, the transporter protein SLC6A8 is necessary to actively transport creatine into the cell.

# Physiological Significance
# General Information
Creatine plays a central role in the energy balance of cells, especially in muscle and nerve cells. Its main function is the short-term storage and provision of energy through the phosphate transfer system:
- Storage as Creatine Phosphate
- In cells, creatine is phosphorylated by the enzyme creatine kinase with ATP (adenosine triphosphate) to creatine phosphate (CrP).
- This compound serves as a quick energy reserve in cells with high energy consumption, particularly in muscle and brain cells.
- Regeneration of ATP
- During high exertion (e.g., intense muscle work), ATP is consumed and converted into ADP (adenosine diphosphate).
- Creatine phosphate donates its phosphate to ADP, regenerating ATP – a rapid energy boost for short-term, intense activities.
- Excretion as Creatinine
- Excess creatine is not stored but is broken down into creatinine and excreted by the kidneys in the urine.
- The creatinine level in the blood provides insights into kidney function.
The cellular creatine reserves for a 70 kg body weight are on average 120 g of creatine. The daily breakdown of creatine amounts to approximately 1.7 to 2%. With a net balance of about 2 grams of creatine per day, the cellular creatine reserves are maintained (van de Kamp et al 2014 (opens new window)).
# Cellular Energy Supply
Fig.: Proportions of various cellular energy production processes depending on the duration of exertion (in seconds). The orange area shows the possible energy loss that could exist without the phosphocreatine, which depends on the creatine transporter. Own illustration.
1. ATP Breakdown (orange line):
- This process provides immediately available energy, e.g., for muscle movement, but only lasts for a very short time (a few seconds) until the adenosine triphosphate is depleted.
2. Creatine Phosphate Breakdown (green line):
- The so-called KP breakdown is activated during ATP breakdown and provides energy for about 10 to 30 seconds. After that, its contribution significantly decreases, but it remains active.
- This rapid energy production process is absent or minimally present in creatine deficiency syndromes.
3. Anaerobic Energy Production (blue line):
- Anaerobic processes increase when the KP reserves decrease (after 10 seconds).
- It involves anaerobic glycolysis, which runs without oxygen and produces lactate.
4. Aerobic Energy Production (red line):
- This process becomes increasingly important as the duration of exertion increases.
- It dominates during prolonged exertion (after about 60 seconds) as it is efficient and sustainable but requires more time to provide energy.
# Organic Mechanism
# Importance of the Creatine Transporter for Vital Organs
Organic functions supported by the creatine transporter can be summarized as follows (according to Farr et al 2020 (opens new window)):
- Skeletal Muscle: In muscular energy metabolism, creatine is involved, among other things, as part of the creatine-phosphocreatine system, acting as an energy reservoir in the cell plasma (cytosolic buffer) by regenerating ATP during muscle contraction (Wallimann et al 2011 (opens new window), Béard & Braissant 2010 (opens new window), Wyss & Kaddurah-Daouk 2000 (opens new window))
- Central Nervous System: The creatine transporter performs essential functions for brain cells, enabling higher cognitive functions. The enriched creatine facilitates ATP regeneration and the transport of high-energy phosphate (similar to skeletal muscle). It is also suspected that creatine acts as a neurotransmitter, synthesized in neurons and released depending on the action potential (Béard & Braissant 2010 (opens new window)), as it was found that the creatine transporter is expressed at synaptic end boutons. Possibly, to absorb or recycle the creatine released and unbound in the synaptic cleft as a neurotransmitter (Hanna-El-Daher & Braissant 2016 (opens new window))
- Heart Muscle: Supplies the heart muscle with creatine.
- Embryonic Development: Creatine transporters in the placenta take up creatine for embryonic and fetal development.
- Gastrointestinal Tract: Supplies intestinal epithelial cells with creatine.
- Immune Regulator: Creatine has a regulatory effect on leukocytes and stabilizes the plasma membranes of erythrocytes.
- Kidneys: Creatine transporters absorb creatine from the primary urine.
- Retina: Uptake of creatine in the retina.
- Skin: Creatine supports wound healing and assists in skin protection from UV exposure.

# Quantification of the Expression of the Creatine Transporter in Various Tissues
A clue to the importance of the creatine transporter for different organs comes from the NIH Genotype-Tissue Expression (GTEx). Even though its accumulation in a tissue type does not necessarily indicate the functional importance of the organ, it does provide insight into the relevance of the transporter for the tissue.

The highest median expression of SLC6A8 across all samples occurs in the colon, sigmoid section (88.78 TPM). There is also relatively high expression in other digestive tissues. In most other tissues, expression is lower or highly variable (depending on the individual). The yellow-marked areas in the diagram are expressions of different regions of the brain. The cerebellum shows slightly higher expression than other brain regions. In the cortex and hippocampus, there is wide variation, which could indicate differing expression between individuals.
More about the Brain Regions:
# Special Importance of Creatine for the Central Nervous System
ToDo
Editing of content is still in progress
- Synapse graphic
- Blood-brain barrier graphic
- Explanation
# Synthesis and Expression of the Creatine Transporter

The SLC6A8 gene encodes the sodium- and chloride-dependent creatine transporter. It is located on the X chromosome, specifically in region Xq28. It consists of 13 exons, which contain the genetic information for the synthesis of the creatine transporter. The resulting protein consists of 635 amino acids and has a molecular mass of approximately 70.523 kilodaltons (kD).
The entire DNA sequence of the gene spans about 8.4 kilobases (kb), while the mRNA, which serves as the blueprint for the protein, is about 3.9 kb long.
Fig.: The figure is created with GenCards and Alphafold and illustrates the presumed folding of the protein. Blue signatures represent highly probable structural predictions, while red areas show uncertainties in the actual folding.
# Synthesis and Expression

Fig.: Schematic representation of the synthesis of SLC6A8 and expression as the creatine transporter in the lipid bilayer. Own figure using image sections from (Ulloa-Aguirre et al 2018 (opens new window))
Description of synthesis and expression based on various publications by (Ulloa-Aguirre & Tao 2018 (opens new window)):
1. Gene Transcription (Nucleus)
- The SLC6A8 gene, which encodes the transporter protein, is located in the DNA within the nucleus.
- Transcription factors bind to the promoter region of the gene and initiate transcription.
- RNA polymerase transcribes the DNA into messenger RNA (mRNA) and modifies it for further processing and transport in vesicles in the cytoplasm.
2a. mRNA Export and Translation (Cytoplasm and Rough ER)
- The processed mRNA exits the nucleus through nuclear pores and enters the cytoplasm.
- Ribosomes bind to the mRNA and begin translation.
- The transporter binds the ribosome to the rough endoplasmic reticulum (RER).
2b. In Case of Incorrect Transcription: Degradation and Recycling
- Misfolded or incorrectly assembled molecular chains are retained in the ER and exposed to resident chaperones.
- Attempts are made to correct the folding and stabilize the transporter protein to allow its export from the endoplasmic reticulum.
- If correct folding fails, the misfolded protein is moved to the cytoplasm, where it is degraded by the proteasome (other proteins).
3. Protein Folding and Posttranslational Modifications (ER)
- In the RER, the newly synthesized polypeptide is folded into its intended three-dimensional form with the help of chaperone proteins.
- Posttranslational modifications such as glycosylation (attachment of carbohydrate groups) and formation of disulfide bonds occur.
4. Protein Transport and Sorting (Golgi Apparatus)
- The transporter protein is transported in vesicles from the ER to the Golgi apparatus.
- In the Golgi, further modifications such as glycosylation and lipid attachment refine the protein.
- The Golgi sorts and packages the transporter into vesicles, which bring it to its final destination at the cell membrane.
5. Vesicular Transport to the Plasma Membrane
- The vesicles containing the transporter move along the cytoskeleton (with the involvement of motor proteins like kinesin or dynein) to the plasma membrane.
- The vesicle fuses with the plasma membrane, incorporating the transporter protein into the lipid bilayer (cell wall).
6. Functional Expression at the Cell Surface
- Once the transporter is embedded in the plasma membrane, it is correctly oriented and functional.
- It begins transporting creatine through the cell membrane.
Regulation and Recycling
- The transporter protein can be regulated through phosphorylation, allosteric modulation, or interactions with other proteins.
- If needed, it can be internalized via endocytosis and degraded in lysosomes or recycled back into the membrane.
# Mutation of the Creatine Transporter
A creatine transporter defect that leads to creatine transporter deficiency (CTD) is typically caused by point mutations in the SLC6A8 gene. These mutations can take different forms:
Nonsense Mutation
A nonsense mutation leads to premature termination of the creatine transporter synthesis. This happens when a stop codon is mistakenly inserted into the DNA. During translation through the mRNA, protein synthesis is prematurely terminated.
In CTD, this results in a defective or incomplete transport protein that is broken down internally by the cell’s quality control system (see previous chapter, points 2b and 6). Since it cannot be properly integrated into the cell membrane, the transport function is lost.
Deletion/Insertion (Frameshift Mutation)
Here, either a base is removed from the DNA (deletion) or an additional base is inserted (insertion). These changes can shift the reading frame of the gene, often leading to the production of a defective or nonfunctional protein.
- In many cases, the synthesis of the creatine transporter is prematurely halted (see previous chapter, points 2b and 6).
- Alternatively, the transporter may still be formed and integrated into the cell membrane, but it has structural defects due to misfolding that severely impair or completely block its transport function.
Missense Mutation
In a missense mutation, the exchange of a single base in the DNA creates a mutated codon, which codes for a different amino acid. This can lead to structural and functional changes in the protein.
- Some missense mutations only partially impair the transporter function, so residual activity remains.
- Others cause severe malfunctions or lead to the protein becoming unstable and being prematurely degraded.
Missense mutations can vary in their effects, ranging from harmless to severe, depending on which amino acid is replaced and what function the affected region of the protein has.

Fig.: Schematic representation of a transporter expressed at the lipid bilayer with the visualization of some gene mutations (and the effect of the synthetic chaperone 4-Phenyl-Butyric-Acid.). Own figure.
# Therapeutic Approaches
# Oral Administration of Creatine Monohydrate, L-Arginine, and Glycine
In a cohort study, 10 out of 28 patients who received high-dose oral creatine monohydrate in combination with L-Arginine and Glycine showed clinical improvement, e.g., a reduction in seizures and improvement in muscular symptoms (Dunbar et al 2014 (opens new window), Valayannopoulos et al 2012 (opens new window)). However, various studies have reported ineffectiveness or lack of efficacy of this treatment approach in increasing brain creatine levels (Jaggumantri et al 2015 (opens new window), Valayannopoulos et al 2012 (opens new window), van de Kamp et al 2011 (opens new window), Fons et al 2008 (opens new window), Bizzi et al 2002 (opens new window)). It should be noted that the combined administration of L-Arginine and Glycine has caused hyperhomocysteinemia in some patients (Villar et al 2011 (opens new window)). High doses of L-Arginine over a prolonged period may expose patients to potential toxicity due to increased nitric oxide synthesis (Valayannopoulos et al 2012 (opens new window)).
In a heterozygous patient with severe epilepsy, the administration of creatine combined with arginine and glycine resulted in complete resolution of seizures (Mercimek-Mahmutoglu et al 2010 (opens new window)).
The main reason for the lack of increased cerebral creatine is discussed to be that, during creatine transport across the blood-brain barrier (BBB), up to three or five membranes must be passed (with or without astrocytes) (Fernandes-Pires & Braissant 2022 (opens new window)), which cannot be crossed, or only crossed in insufficient quantities, without a functional transporter protein: from the microcapillary endothelial cells at the BBB to the surrounding astrocytes and to the brain cells in the CNS parenchyma (e.g., neurons and oligodendrocytes).
# Development of Lipophilic Creatine Analogues
Creatine analogues are very similar to the basic substance creatine and thus have comparable chemical properties. Some lipophilic creatine derivatives are believed to have good permeability through the lipid bilayer of the cell membrane. This could make it possible to increase the creatine content at the organic action sites even without the creatine transporter. The mechanism of action is depicted in the above figure under point 2. Lipophilic creatine analogues could therefore emerge as a promising drug candidate (Trotier-Faurion et al 2013 (opens new window)).
# Conjugation of Creatine with Salts
By conjugating creatine with salts, creatine uptake can occur via other transporters. This is depicted in the above figure under point 3.
# Pharmacological Chaperones
Pharmacological chaperones are small molecules that prevent/correct the misfolding of the mutated protein, facilitate its translocation to the correct cellular location, and may enable it to regain its activity. Therefore, they can be a therapeutic approach for patients with misfolding mutations of SLC6A8 variants, working during the folding of the transporter in the endoplasmic reticulum or correcting misfolded creatine transporters at the cell membrane (see the above figure, point 4). For some mutations that led to misfolding of the transporter, experiments with 4-Phenyl-Butyric-Acid (4-PBA) and heat shock proteins have been conducted (Farr et al 2020 (opens new window)) to correct misfoldings. Although the exact mechanisms of 4-PBA are still largely unknown, studies with the synthetic chaperone 4-PBA fundamentally show that misfoldings in the SLC6A8 protein can be corrected, provided the mutation variant is due to protein-folding errors in the endoplasmic reticulum (El-Kasaby et al 2019 (opens new window)). The reason for this is that 4-PBA acts in the endoplasmic reticulum and can influence the folding of the transporter protein there.
# Gene Therapy
Currently, several approaches are in development based on adeno-associated viruses (AAV). These viruses are already used as viral vectors in gene therapy and have proven to be one of the most commonly used and safest vectors (Fernandes-Pires & Braissant 2022 (opens new window)). In regard to the creatine transporter defect, the special advantages of AAVs are that they can be easily manipulated to introduce a transgene for gene transfer into cells/nuclei, and they can infect neurons with the desired gene sequence. This could enable the establishment of functional transporter proteins in brain cells, allowing patients to accumulate sufficient cellular creatine.
# Treatment of Patients
Relevant Literature
Please refer to the medical literature and consider recent updates regarding the oral administration of creatine monohydrate with supplements. There may be newer procedures available. Consult medical experts or contact us for further information and referrals.
